Activated carbon adsorption is a captivating process that plays an essential role in various applications, from environmental remediation to water treatment and air purification. This porous material, with its extensive surface area and highly porous structure, has become a darling of both industry and academia. But what exactly is activated carbon, and how does the adsorption process work? Furthermore, how does biochar, a carbon-rich material produced from biomass, relate to this intriguing phenomenon? Let’s delve into the intricacies of activated carbon and uncover the synergy with biochar.
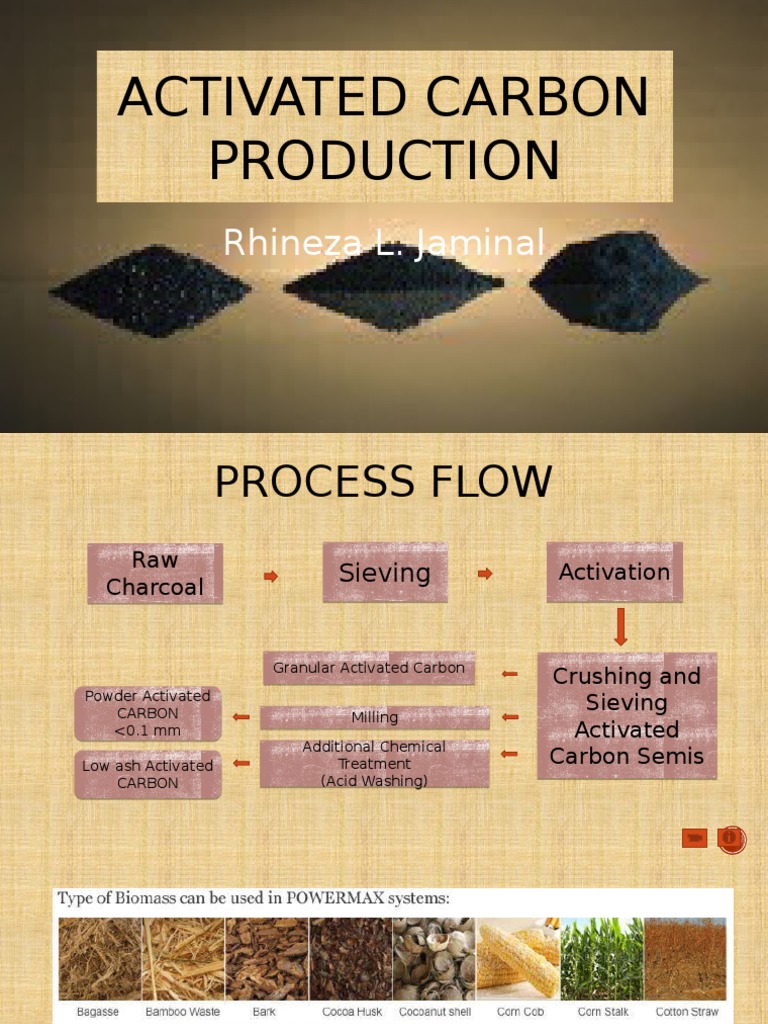
At its core, activated carbon is derived from organic materials such as wood, coconut shells, or coal. The term ‘activated’ refers to the treatment process that enhances its adsorptive properties. Through high-temperature pyrolysis, the carbon source undergoes a transformation that creates an abundance of micro-pores and enhances its surface area, which can exceed 3000 square meters per gram. This virtually infinite surface area allows for the effective adsorption of various contaminants, making it a quintessential agent in purifying air and water.
Adsorption, the primary mechanism by which activated carbon captures contaminants, is distinct from absorption. While absorption refers to one substance being taken up into the interior of another, adsorption is a surface phenomenon. Molecules of gases or liquids adhere to the surface of the activated carbon grains, primarily due to intermolecular forces. This includes van der Waals forces and chemical bonding. Such a relationship surfaces in a myriad of scenarios, including the removal of pollutants, toxins, and odors, as well as the capture of volatile organic compounds (VOCs).
The mechanics of adsorption can be understood through the following stages: first, diffusion—a crucial preliminary stage where the contaminant molecules migrate to the surface of the activated carbon. Next, is the adhesion phase, where covalent or electrostatic interactions facilitate the attachment of these molecules. Finally, the equilibrium state is reached when the rate of adsorption equals the rate of desorption, ensuring an effective capture of pollutants without immediate release back into the environment.
The capacity of activated carbon to remove specific contaminants is determined by several pivotal factors. These include the physical characteristics of the carbon itself, such as pore size and surface chemistry, as well as the nature of the target pollutant. For instance, larger organic molecules may require larger pores for effective adsorption, while charged species might interact differently depending on the chemical makeup of both the adsorbent and the adsorbate. Understanding these dynamics is not merely academic; it is critical for optimizing the adsorption efficiency in real-world applications.
Furthermore, the regeneration of activated carbon presents an intriguing aspect of its use. After a period of effective adsorption, the carbon can become saturated and lose its efficacy. However, this material can often be reactivated through methods such as thermal regeneration or chemical washing, allowing it to regain its adsorptive prowess. This regenerative potential not only reinforces the sustainability aspect of activated carbon usage but also renders it economically viable, especially for large-scale industrial applications.
As we explore the fascinating realm of carbon materials, biochar emerges as a prominent counterpart to activated carbon. Often considered a byproduct of pyrolysis, biochar is formed through the thermal decomposition of organic matter in a low-oxygen environment. This carbon-rich material is witnessing growing interest for its potential applications in agriculture, soil enhancement, and carbon sequestration.
The link between activated carbon and biochar lies in their shared genesis. Both materials possess unique porous structures, but unlike activated carbon, biochar is typically produced at lower temperatures and does not undergo the same level of activation. Consequently, while biochar can adsorb certain contaminants—particularly in soil and water—it may not achieve the same efficacy as activated carbon in industrial applications. Nevertheless, its agronomic benefits, such as enhancing soil fertility, improving water retention, and providing habitat for beneficial microorganisms, make biochar a valuable asset in sustainable practices.
Moreover, biochar showcases potential in mitigating greenhouse gas emissions. When incorporated into the soil, it exhibits a remarkable ability to sequester carbon for extended periods, effectively removing CO2 from the atmosphere. This inherently forms a dual solution for both air quality management and climate change mitigation, as utilizing biochar can offer both agricultural and environmental benefits.
The integration of activated carbon and biochar into a unified framework opens up an array of innovative applications. For example, combining biochar’s soil enhancement properties with the adsorptive capabilities of activated carbon could lead to synergistic effects, resulting in improved agricultural yields and reduced contaminant levels in agricultural runoff.
In conclusion, the world of activated carbon adsorption is as complex as it is critical in addressing contemporary environmental challenges. With its ability to purify water and air while engaging in remarkable chemical interactions, activated carbon offers a multifaceted approach to pollutant removal. Concurrently, the rise of biochar presents new opportunities for innovative sustainable development. The interplay between these two carbon materials reveals not only their individual significance but also their collective potential for a greener future. Each application extends beyond mere functionality; it hints at a deeper interconnectedness within natural cycles, thus revealing the profound relationship between material science and ecological stewardship.